
Anabolic-androgenic steroid effects on libido (Part 2: Women)
Share
Definitions
Libido: sexual desire or sexual interest that arises out of central arousal and response, manifested as thoughts about sex accompanied by genital response. [1].
Sexual function: a broader concept that encompasses measures of libido (e.g., Male Sexual Health Questionnaire: sexual desire [comprehensive]), as one aspect or component.
Anabolic-androgenic steroids (AAS), or androgens generally enhance libido.
Introduction
This is the second article in a two-part series on the topic of androgen effects on libido in men and women. This second article will focus on androgen effects on libido in women.
In part one of this series, Anabolic-androgenic steroid effects on libido in men, we discussed how testosterone (T) and its 5α-reductase product, DHT, exerts a clear organizing & activating effect on sexual behavior, including sexual desire (libido). Here, we focus on androgen effects on libido in women, where the effects can be less consistent and robust, despite, interestingly, a greater behavioral responsiveness to androgen in females. [1]. [2].
Women and men differ broadly with respect to libido. Few would argue, despite some variation and the existence of relative outliers, that men are not characteristically more virile than women. And yet, despite a broad consensus between biological and biomedical researchers, as well as the lay public, that testosterone is implicated in sex differences in libido and indeed enhances libido, there is surprisingly conflicting evidence in the literature on this subject, particularly with respect to differences in and between women.
Threshold effect
While in men (despite strong evidence to the contrary), the prevailing medical view is that men are subject to a threshold effect of testosterone on behavior (including on libido); this model does not apply to women. Women, then, are not subject to any apparent ceiling or floor effect, above which T concentrations do not contribute to increased libido, or below which symptoms of androgen deficiency arise and result in pathology, respectively. This is partly due to the lack of established reference ranges for normal or healthy T concentrations and to lack of adequate assay sensitivity to detect the low concentrations that might constitute the floor for testosterone concentrations (as it would be very low).
More interestingly, though, is the lack of a theoretical upper limit, or ceiling, for behavioral effects of androgen in women.
Points of apparent contradiction in testosterone-libido effects in women
Several lines of apparently contradictory responses of libido to testosterone exist in women versus their male counterparts.
Women are strongly influenced by so-called affect (mood, well-being, and energy) on libido. Given the potent effects of mood on libido in women, depression, anxiety, and stress increase the adrenal output of testosterone and other adrenal hormones in women; whereas in men, these negative mood states decrease testicular output of testosterone (that adrenal output cannot overcome). [1]. And yet, a significant minority of men demonstrate a paradoxical increase in libido in states of anxiety, and even depression; whereas women do not apparently demonstrate this paradoxical enhancement of libido.
A very basic confound that presents difficulty in studying the effects on libido by androgen is that female genital response (vaginal secretion) is fundamentally dissociated from cognition, or perception of sexual arousal. It is frequently observed that genital response is not perceived as increased sexual arousal in women; whereas in men, the erectile response unmistakably enhances libido, feeding back to further modulate drive and function (contributing to maintenance of the erection). [1]. And yet, treatment of sexual dysfunction in women by improving symptoms of vaginal dryness may be second only to improving affect (mood, well-being, and energy) in therapeutic efficacy.
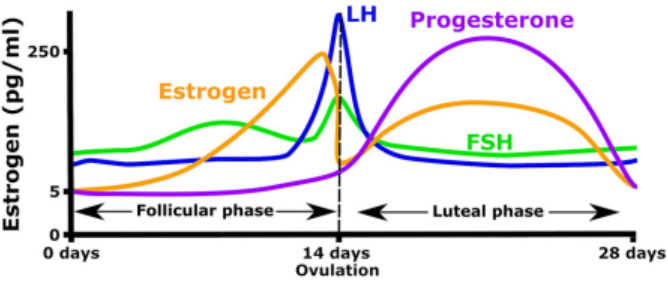
Still, the effect of hormones on female libido is undeniably powerful. During the ovulatory-menstrual cycle (OMC), the late follicular phase just prior to ovulation is characterized by high LH & T, low progesterone & increasing E2 (from 5 pg/mL in the early follicular phase to a peak of 200 – 500 pg/mL just before ovulation) that declines abruptly, and concomitantly increasing FSH. [3]. Women consistently report that libido rises steadily in the week prior to ovulation, and peaks around the time of ovulation, only to be followed by a precipitous decline over the subsequent week. [6]. During the adult OMC, primarily ovarian T production follows a cyclical pattern where T levels increase during the follicular phase and are at a peak approximately for the middle 1/3 of the OMC, declining during the final 1/3 (luteal phase) to reach the nadir during the first few days of the next follicular phase. Within the middle 1/3, T levels may be relatively steady or appear in peri-ovulatory peaks. [1]. Here, T may be a controlling influence; or, E2, LH & FSH could be major factors in enhanced libido.
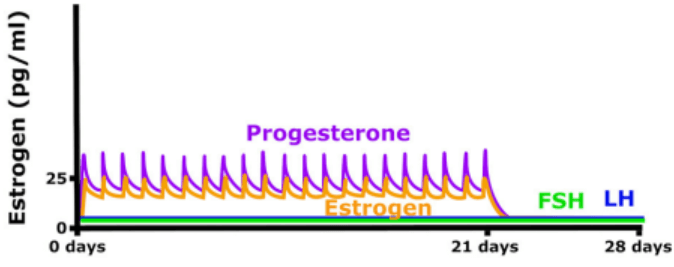
Oral contraceptives should theoretically reduce libido by reducing free T (exogenous estrogen, often plus a progestin) increases SHBG (that binds T E). Progestins generally exert antiandrogenic effects, are associated with decreased AR expression, and combination with estrogens increases PR expression. [4]. [5]. While oral contraceptives are associated with decreased libido with some significance, the effect is not robust and exceptions are seen. These are usually attributed to qualitative differences in the population of oral contraceptive-using women, that may have lower trait anxiety and therefore more positive affect (mood, well-being, and energy), reduced prevalence of sexual problems, and generally more permissive sexual behaviors and attitudes (perhaps even higher base-line endogenous testosterone).
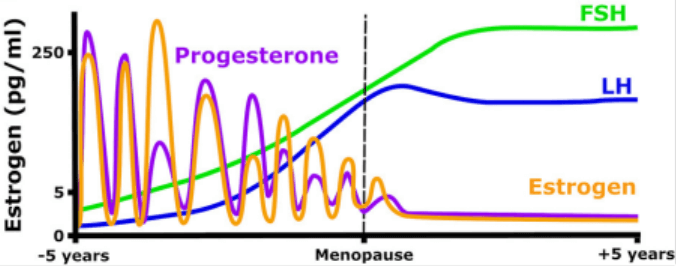
The clearest effects of testosterone enhancement of libido in women comes from HRT (hormone replacement therapy) administration to women without sexual problems. This still attaches with some confusion, however. The population of women that are prescribed HRT is generally perimenopausal (either transitioning towards reduced, experiencing diminished, or eventual total practical abolishment of E2 & progesterone secretion that characterizes menopause). Ovariectomized female rats given E2 (estradiol benzoate) alone renders them moderately sexually receptive, whereas treatment with E2 + progesterone renders them fully sexual receptive and proceptive. [6]. HRT for women administered as estrogen + testosterone (and typically, supraphysiological testosterone) consistently increases libido in women without sexual problems. Whereas exogenous estrogen reduces free testosterone & bioavailable estrogen, in fact, by increasing SHBG (that binds T E), exogenous testosterone increases free testosterone & bioavailable estrogen by decreasing SHBG and by T aromatization to E2. The question arises, what is the contribution – positive, negative, or neutral – of increased bioavailable estrogen on libido? And in women versus men?
Also, HRT typically enhances mood, well-being, and energy: given the dominating influence of affect on libido in women; are the effects of E + T merely indirect, by enhancing these factors?
Further, with long-term administration of HRT to, typically menopausal and postmenopausal women, there is eventually (after at least several months) a decrement in the enhancement to libido. This has been variously attributed to three potentially confounding factors:
- That it is the increase per se or degree of change in, rather than the absolute quantity of, testosterone concentrations that determine libido enhancement; once changes to T concentrations taper off, it would seem that libido similarly tapers off or abates.
- That there is an age-related decrease in AR sensitivity (in women as well as there is in men), and as these women grow older, their sensitivity abates.
- That there is desensitization to the behavioral effects (i.e., libido) of androgen. Bancroft and colleagues have proposed The Desensitization Hypothesis as a hypothetical framework to explain apparent contradictions in testosterone-libido effects in women. [1]. [2].
The Desensitization Hypothesis
A theoretical attempt to explain sex differences in androgen effects on libido. [1]. [2].:
- The greater variability in the sensitivity to androgens in women could result from a greater genetic variability in women, on the grounds that in women, behavioral responses to gonadal steroids is less crucial than is the case with men.
- One of the consequences of the far greater T levels in men is that they show masculinizing effects, such as increased growth and muscle bulk, dependent on the peripheral anabolic effects of T. It has been postulated that if males were as sensitive to the CNS effects of T as females, then the behavioral effects of these masculinizing levels would be maladaptive (see Part 1 of Series on Anabolic-androgenic steroid effects on libido in men and women: Androgen Receptor Function in CNS (males)). Hence, there is a need in the male to reduce responsiveness to androgen effects in the brain.
- Exposure to substantially higher T levels during fetal development and also during the first few weeks postnatally [the perinatal surge] could be responsible for desensitizing the CNS to T effects in the male. Such desensitization would presumably act on the genomic level rather than the receptor stage of hormone action… and in the short-term, both T & DHT exposure results in upregulation of AR. A consequence of such desensitization in the male would be that genetically determined variations in CNS receptor responsiveness to T would be “flattened out,” as well as allowing much higher levels of T from puberty onward without hyperstimulation of CNS mechanisms.
- With no such desensitization in females, the basic genetic variability would be more evident, at much lower levels of T, and manifested as greater variability in behavioral responsiveness, demonstrated from early adolescent development onward.
- Evidence from studies of women with congenital adrenal hyperplasia (CAH), particularly the salt-losing variety associated with higher levels of T during fetal development, shows not only some degree of masculinization of behavior but also low levels of sexual interest. Although in such cases there are a number of factors which could impair normal sexual development, this evidence is consistent with there being some degree of desensitization to the high fetal levels of T, which fall and remain low after birth when the CAH is treated.
- An interesting question is whether this hypothetical desensitization mechanism is an “organizing effect” of high T that is only operative during early development, or whether such suppression is possible if exposure to high levels occurs later in development. Evidence of “tolerance” to [behavioral effects on libido of] supraphysiological T was reported in several of the HRT studies reviewed earlier. This suggests that such desensitization might occur later in life also, at least to some extent… However, there may alternately be a decline in AR sensitivity in women as they age comparable to that found in men.
Conclusion
Whereas endogenous androgens (T & DHT) exert a clear effect in men on libido, in women T’s effects are less clear despite greater behavioral responsiveness, due to greater sensitivity to the effects of mood, energy, and well-being, and to the vagaries of a complex interplay between the ovulatory-menstrual cycle and behavior.
Supraphysiological androgens generally augment libido in men (even those that are normal and healthy) and women, but chemical modifications to androgens can affect whether particular androgens exert an enhancing or even suppressing (e.g., nandrolone) effect on libido in men and women alike.
It is imperative that the reader understand the important role of dopamine and the excitatory system and the modulation of libido by steroid hormones via dopamine circuitry. Refer to Dopamine and libido (from Part 1 of Series on Anabolic-androgenic steroid effects on libido in men and women) for insight into this aspect of libido in humans (in men and women alike). While it is difficult to disentangle the effects of testosterone on sexual behavior in women from those of estrogens, that exogenous testosterone increases the bioavailability of, there is indisputable evidence that testosterone at supraphysiological doses augments libido in women free from sexual problems.
The Desensitization Hypothesis is a theoretical framework to explain the apparent contradictions between the sexes (as well as between women) in the effects of androgen on libido.
Men are simple, testosterone clearly governs sexual function and libido (with some influence from aromatization to estradiol, particularly in CNS and brain). Women are more multifaceted in the dynamics of their hormonal milieu, and to draw any inferences about hormonal effects on female behavior requires a nuanced theoretical model, informed observer, and a granular lens, to even attempt rational description.
